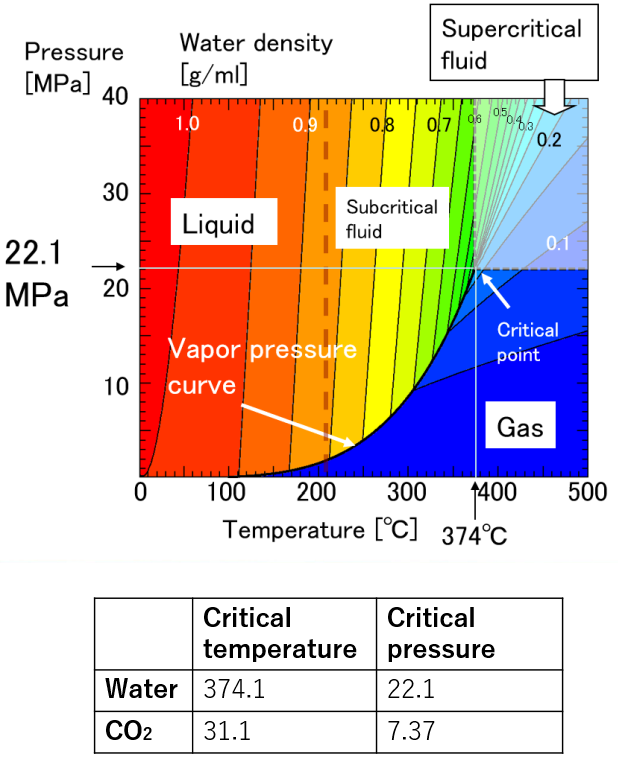
A supercritical fluid is a unique fluid that exceeds the limit temperature and pressure at which gas and liquid can coexist, and exhibits properties different from ordinary gas and liquid. This limit is called the critical point. For water, the critical point is a pressure of 22.1 MPa and a temperature of 374 ° C. (See the right figure)
At room temperature, 70% of water molecules are hydrogen-bonded to four water molecules and have the same crystal structure as ice. The remaining 30% have liquid properties because they have 3 or less hydrogen bonds and do not form a crystal structure. The proportion of crystal structure decreases as the temperature rises and the kinetic energy of the molecule increases. Above the boiling point, the water molecules are completely separated and become a gas. Water remains liquid under high pressure, but as the temperature rises, the specificity of water at room temperature decreases. As shown in the figure below, the permittivity and ion product change significantly. The permittivity decreases with temperature and becomes equivalent to organic solvents. As can be seen from the value of the ion product, water is most likely to dissociate at around 250 ° C, but at higher temperatures it is less likely to dissociate rapidly, making it easier to contribute to the hydrolysis reaction.
See also this page.
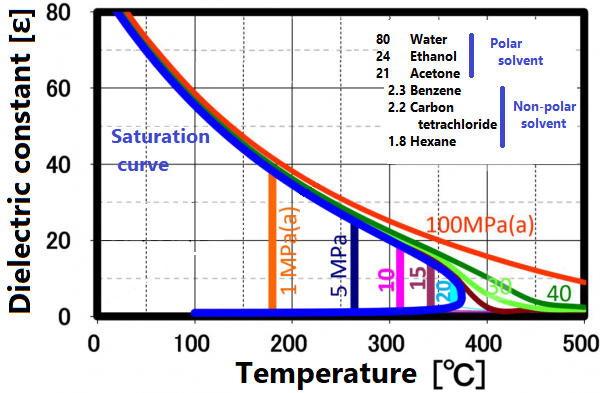
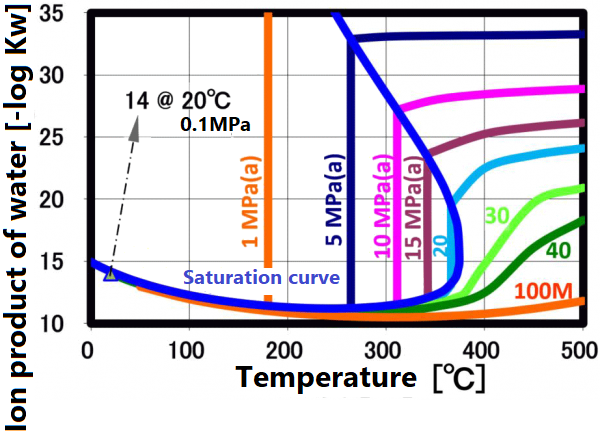
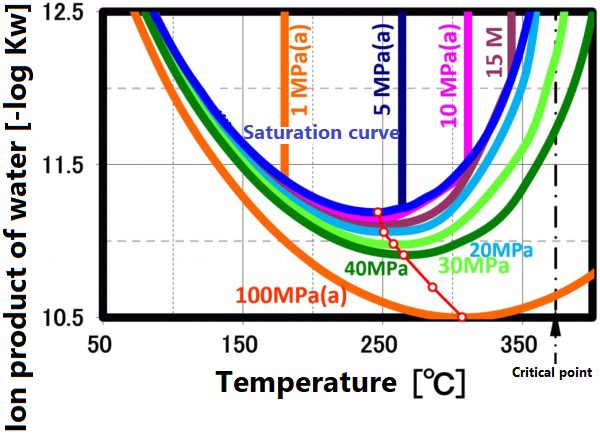
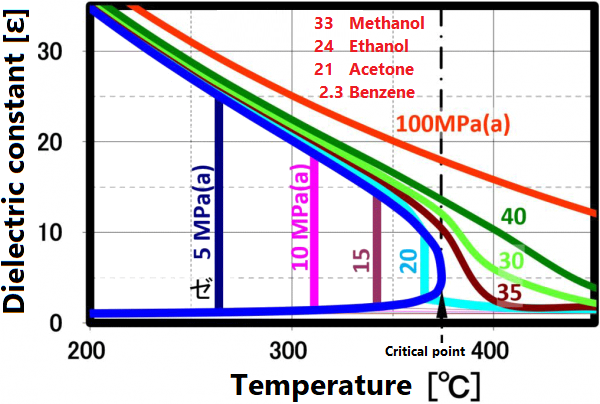
Dielectric constant
The permittivity reflects the polarity of the solvent. Liquids and supercritical water have a reduced permittivity as the temperature increases. In other words, water having a relative permittivity of 80 at room temperature drops to the same dielectric constant as methanol and ethanol at 200 °C and acetone at 300 ° C. When the temperature rises above the critical point, it exhibits a relative permittivity equivalent to that of non-protic solvents such as hexane. Mixing non-protic solvents with supercritical water is known to result in a homogeneous phase, which is brought about by this reduction in permittivity. Utilizing this property, it is possible to dissolve low-polarity substances in water for supercritical treatment.
Dissociation constant (ion product)
The dissociation constant Kw of water is 10⁻¹⁴(mol/kg)² at 20 °C. On the other hand, in sub-critical water, the ion product increases significantly as the temperature increases up to about 250 °C (the vertical axis in the figure is a negative logarithm, so the value decreases as the ion product increases). The larger ion product indicates that the acidic and/or alkaline properties become stronger. And this temperature range is suitable for taking advantage of these properties.
This ion product reaches its maximum around 250 °C (Ion product is about 1000 times that of water at room temperature), and its temperature gradually increases as the pressure increases. At higher temperatures, the ion product decreases rapidly due to density decrease, approaching gas-like properties. Therefore, for example, when hydrolyzing a condensation polymer, it is more efficient to use subcritical water at around 250 °C and a pressure of about 10 MPa rather than supercritical water. In high temperature regions such as supercritical conditions, synthesis involving radical reactions is suitable.
