About supercritical water
What is supercritical water?
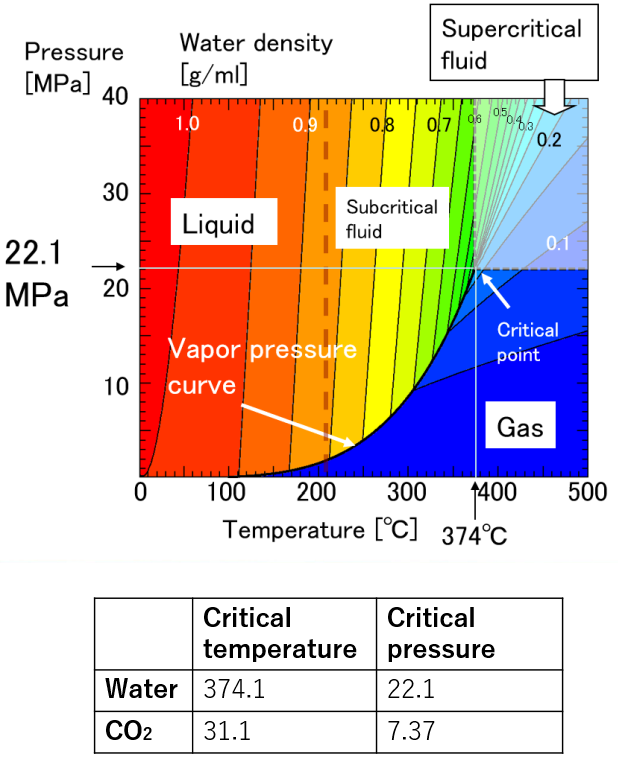
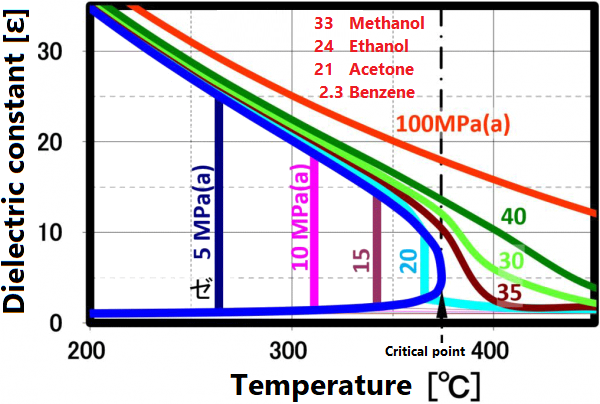
A supercritical fluid is a unique fluid that exceeds the limit temperature and pressure at which gas and liquid can coexist, and exhibits properties different from ordinary gas and liquid. This limit is called the critical point. For water, the critical point is a pressure of 22.1 MPa and a temperature of 374 ° C. (See the right figure)
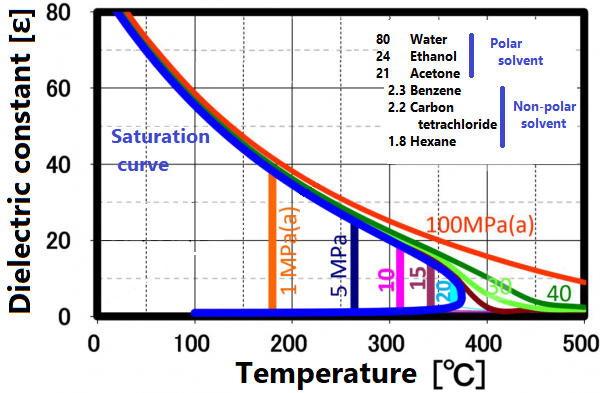
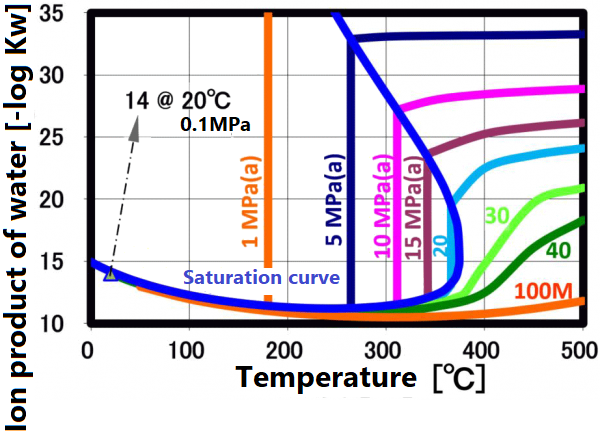
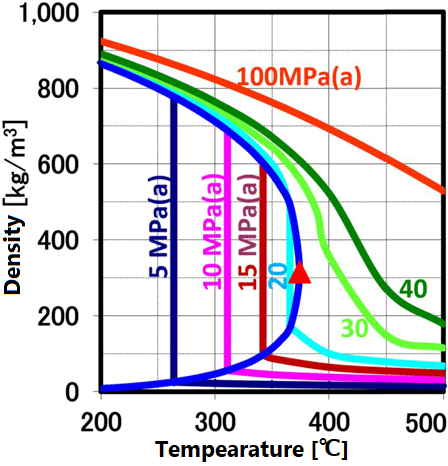
At room temperature, 70% of water molecules are hydrogen-bonded to four water molecules and have the same crystal structure as ice. The remaining 30% have liquid properties because they have 3 or less hydrogen bonds and do not form a crystal structure. The proportion of crystal structure decreases as the temperature rises and the kinetic energy of the molecule increases. Above the boiling point, the water molecules are completely separated and become a gas. Water remains liquid under high pressure, but as the temperature rises, the specificity of water at room temperature decreases. As shown in the figure below, the permittivity and ion product change significantly. The permittivity decreases with temperature and becomes equivalent to organic solvents. As can be seen from the value of the ion product, water is most likely to dissociate at around 250 ° C, but at higher temperatures it is less likely to dissociate rapidly, making it easier to contribute to the hydrolysis reaction.
See also this page.
Dielectric constant
The permittivity reflects the polarity of the solvent. Liquids and supercritical water have a reduced permittivity as the temperature increases. In other words, water having a relative permittivity of 80 at room temperature drops to the same dielectric constant as methanol and ethanol at 200 °C and acetone at 300 ° C. When the temperature rises above the critical point, it exhibits a relative permittivity equivalent to that of non-protic solvents such as hexane. Mixing non-protic solvents with supercritical water is known to result in a homogeneous phase, which is brought about by this reduction in permittivity. Utilizing this property, it is possible to dissolve low-polarity substances in water for supercritical treatment.
Dissociation constant (ion product)
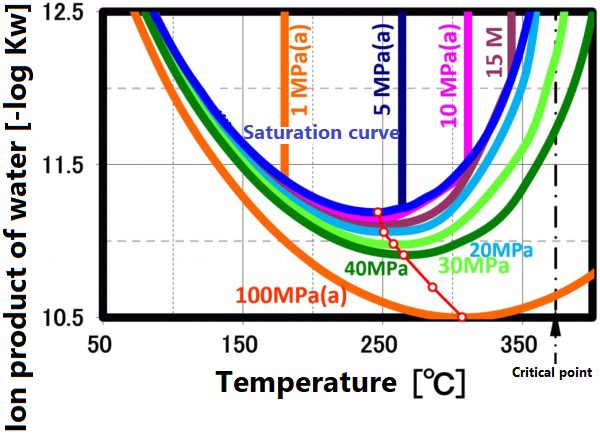
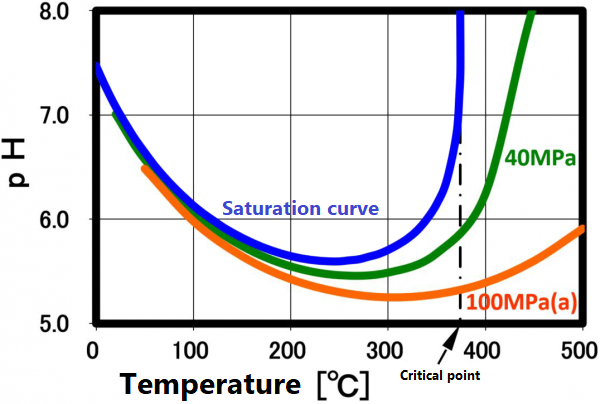
The dissociation constant Kw of water is 10⁻¹⁴(mol/kg)² at 20 °C. On the other hand, in sub-critical water, the ion product increases significantly as the temperature increases up to about 250 °C (the vertical axis in the figure is a negative logarithm, so the value decreases as the ion product increases). The larger ion product indicates that the acidic and/or alkaline properties become stronger. And this temperature range is suitable for taking advantage of these properties.
This ion product reaches its maximum around 250 °C (Ion product is about 1000 times that of water at room temperature), and its temperature gradually increases as the pressure increases. At higher temperatures, the ion product decreases rapidly due to density decrease, approaching gas-like properties. Therefore, for example, when hydrolyzing a condensation polymer, it is more efficient to use subcritical water at around 250 °C and a pressure of about 10 MPa rather than supercritical water. In high temperature regions such as supercritical conditions, synthesis involving radical reactions is suitable.
Trial test
It is possible to visit for the experiment. Various treatments using subcritical water and supercritical water are possible for the test. For example, nanoparticles can be synthesized by hydrolysis, dehydration, etc. (organic modified particles can also be synthesized). The apparatus is capable of flow reactors (continuous reaction and continuous recovery) in addition to batch reactors. See Q.6 for details.。
In the standard test, the upper limit temperature is 450 ° C and the upper limit pressure is 30MPa. By setting up the device, it is possible to test with an upper limit temperature of 600 °C and 27 MPa.
The ion product of subcritical water and supercritical water is shown in A.0. As shown in the right figure, the pH decreases with increasing temperature and reaches 5.6 at 250 °C. It increases as the temperature increases further, reaching 8 at the critical point, which is equivalent to that of methanol at room temperature.
Even if the pH changes, the water itself is neutral, but piping materials such as SUS are exposed to conditions that are easily corroded. Please contact us for more information.
It is possible.
In some cases, subcritical water which has lower temperature and pressure is more suitable than supercritical water. Please also refer to Q.2 and trial test examples.
Not only water but also solvothermal and supercritical alcohol can be tested. It is also possible to perform tests in which carbon dioxide (reported to be miscible with water at pressures above 30 MPa and temperatures above 360 ° C) is mixed with water.
The following systems are available.
Supercritical hydrothermal treatment system in a continuous flow
MOMI-cho®mini
Maximum pressure 30MPa, maximum temperature (before mixing) 450℃, water feeding rate 10ml/min
By mixing an aqueous metal salt solution with high-temperature water, fine particles can be synthesized by hydrolysis and dehydration.
In addition, it is possible to modify the organic group on the surface by adding a slurry of particles.
Although it depends on the feeding rate of the raw material, a typical production amount is about 2 to 3 g / Hr.

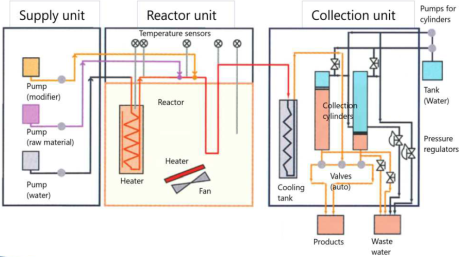
Batch reactors
Maximum pressure 30MPa, maximum temperature 400℃, volume 50ml(φ19). Pressure control is possible.
High pressure slurry pump
Slurries and high viscosity liquids cannot be fed with a plunger pump. In that case, this device is required, where a stirrer is used to prevent the slurry from settling. There is a single cylinder type (right photo: 30MPa, 10ml / min) and a double cylinder type (30MPa, 100ml / min) that can send liquid without interruption. Also, when feeding biomass or magnetic particles, a forced stirring method is required. In that case, please contact us.

There are no particular restrictions as long as it can be done using this system. The following are typical examples.
- Nanoparticle synthesis (rapid heating, slow heating), organic modification
- Long-term batch test, sub/supercritical mixed reaction test (slow-speed heating), sub/supercritical water test in the presence of gas (slow-speed heating)
- Material corrosion test, material surface treatment test, waste treatment test
Applications
The following are listed.
Some examples of sub/supercritical water treatment
| Particle synthesis | a) Hydrothermal synthesis of metal oxide: See Q9 Barium titanate BaTiO₃, zirconia ZrO₂, cerium oxide CeO₃, optical materials (TiO₂, ZnO, ITO), etc. b) Organic modification on the surface of inorganic particles: see Q10 Oleic acid-modified cobalt blue nanoparticles, thermal conductive filler |
| Recycle | a) Chemical recycling of organic polymers: Converts dehydration condensation compounds into monomers. See Q11. b) Others: Recycling of wood resources. See Q11 Conversion of fuels such as chlorinated solvents, recovery of carbon fibers from CFRP, etc. |
| Decomposition | a) Supercritical hydroxyl decomposition: Decomposes hazardous organic chlorine compounds such as PCBs and dioxins with oxygen. b) Lightening: Pipeline fluid transportation by lightening high-viscosity oil sands, extra-heavy oil, etc. See Q12. c) Gasification: Conversion from biomass/organic waste to fuel gas, etc. |
Zirconia nanoparticles ZrO₂: Fine particle powder with a primary particle size of 10 nm or less. The main crystal structure is tetragonal. Spherical particles with a secondary particle size of 19 nm
Oleic acid-modified cobalt blue nanoparticles CoAl₂O₄: It has been reported that the surface is covered with organic molecules, so it has a high affinity with organic materials and mixes uniformly with polymers. (20200413 accessed at https://www.wpi-aimr.tohoku.ac.jp/ajiri_labo/research/research_002.html)
Water is a substance that is abundant on the earth, but it has very unique physical characteristics compared to other materials. For example, a) It is suitable for a heat medium because it has a large specific heat. b) Since it is a non-linear molecule and has polarity, it has a large dielectric constant (relative permittivity 80) and dissolves electrolytes well. c) Due to its polarity, hydrogen bonds work between water molecules, so it is a liquid at room temperature for its small molecular weight. d) The water itself is partially dissociated. See also Q.0.
As the temperature of water is raised under pressure, the permittivity and ion product change significantly as shown in Q.0, and the properties are completely different from those of water at room temperature, making it possible to create new materials. In particular, the ability of supercritical water to be miscible with organic solvents such as oil has great advantages in chemical reactions and material synthesis. At room temperature, a special method has been required so far in order to react using liquids that do not mix with each other. In the supercritical state, it is possible to proceed the reaction by setting the temperature and pressure.
The advantages are summarized below.
| Easy solvent removal | Physical properties can be controlled | High reactivity at high temperatures | Characteristics of both gas and liquid |
| ・The dielectric constant can be adjusted by setting the temperature and pressure: water and organic compound can be mixed ・The ionic product of water is also variable: hydrolysis or radical reactions are possible |
Composite metal oxides can be synthesized. Particle synthesis and organic modification are possible at the same time: By modification, it can be dispersed in solvents or resins at high concentration and low viscosity. |
・Low molecular weight, low viscosity ・Low surface tension ・High diffusion/high permeability ・Density fluctuation/clustering |
When an aqueous metal salt solution is heated, hydroxides and oxides become more thermodynamically stable than in the ionic state. Hydrothermal synthesis reactions can be thought of as hydrolysis and dehydration reactions. Particle synthesis of oxides and hydroxides by hydrothermal treatment takes advantage of this equilibrium shift.
MAx + xH₂O = M(OH)x + xHA、M(OH)x = MOx/2 + x/2H₂O
Where A is an anion like Cl⁻, NO₃⁻, or SO₄⁻.
Followings are the typical examples.
| Raw material | Particle | Diameter [nm] | Temperature [℃] | Pressure [MPa] | Forms |
| Al(NO₃)₃ | AlOOH | 50-600 | 250-400 | 25~40 | Hexagonal plate |
| Ce(NO₃)₃ | CeO₂ | 20-300 | 250-400 | 30 | Octahedron |
| Co(NO₃)₃ | Co₃O₄ | ~100 | 400 | 35 | Octahedron |
| Fe(NO₃)₃ | α-Fe₂O₃ | ~50 | 400 | 35 | 球状 |
| Gd(NO₃)₂ | Gd(OH)₃ | ~20 | – | – | |
| Ni(NO₃)₂ | NiO | ~200 | 400 | 35 | Octahedron |
| Ni(OAc)₂+H₂ | Ni | ~500 | – | – | |
| Ti(SO₄)₂ | TiO₂ | ~20 | 400 | 35 | Spherical |
| Zn(NO₃)₂ | ZnO | ~20 | – | – | |
| ZrOCl₂ | ZrO₂ | ~10 | 400-490 | 30 | Spherical |
| Raw material | Particle | Diameter [nm] | Temperature [℃] | Pressure [MPa] | Form |
| Fe(NH₄)₂H(C₆H₅O₇)₂ | Fe₃O₄ | ~50 | 400 | 35 | Spherical |
| Al(NO₃)₃, Co(NO₃)₂ | CoAl₂O₄ | ~10 | – | – | |
| Ba(NO₃)₂, Fe(NO₃)₃ | BaO₆Fe₂O₃ | 50-300 | 400 | 30 | |
| Ba(NO₃)₂, Ti(SO₄)₂ | BaTiO₃ | 50-1,000 | – | – | |
| Co(OAc)₂, Fe(OAc)₂ | CoFe₂O₄ | 40-70 | 200-400 | 25 | |
| LiOH,Co(NO₃)₂ | LiCoO₂ | 50-1000 | 300-400 | 100 | |
| Li salt, phosphate, etc | LiFePO₄ | 350 | 450 | – | |
| LiOH,Mn(NO₃)₂,H₂O₃ | LiMnO₄ | 20-40 | 400 | 30 | |
| Ni(OAc)₂, Fe(OAc)₂ | NiFe₂O₄ | 30-40 | 190-400 | 25 | |
| Fe(NO₃)₃, Sr(NO₃)₂ | SrFe₁₂O₁₉ | 200 | 400 | 30 |
References: Journal of the Soceity of Inorganic Matericals, Japan 12,429 (2005) Table 1
高圧力の科学と技術 vol.20,3 (2010), 超臨界流体とナノテクノロジー, CMC publishing, p103 table.1 etc. (in Japanese)
Nanoparticles shown in Q.9 can be synthesized by using high temperature and high pressure water. At the same time, the surface can be organically modified. The organic substances used are mainly those with a carboxyl group (such as alkylcarboxylic acids). Here are some examples.
Shape control / crystal plane control
CeO₂ nanoparticles
a) Unmodified: octahedron-shaped nanocrystal with (111) surface
b) Moderately modified: cubic nanocrystal with (100) surface (high reactivity)
c) Highly modified: pentadecahedron-shaped nanocrystal
Low viscosity and high concentration dispersion
Alumina with different particle sizes is mixed to increase the volume ratio up to 80% and maintain fluidity.
→ Encapsulant for electronic device packaging
Heat conductive filler used for heat dissipation material
Organic modified Al₂O₃
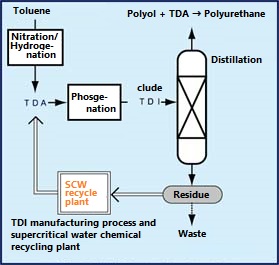
An example of a waste chemical recycling process is to hydrolyze the production residue of TDI (toluene diisocyanate), which is a raw material for polyurethane, with subcritical water to recover TDA (toluenediamine). A chemical recycling plant that converts TDA to TDI started operation in 1998 in Kashima City, Chiba Prefecture, for the first time in the world. The sub-critical water treatment method is an environment-friendly method that can regenerate TDI, which has been incinerated in the past, using only water without using an alkaline catalyst.
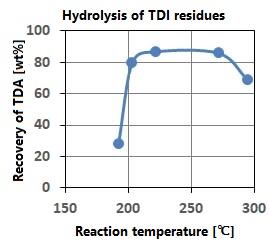
This method utilizes the fact that the ion product of sub-critical water increases significantly and the hydrolysis reaction is likely to occur, as shown in Q.0. As shown in the figure on the right, the TDA recovery rate is high at temperatures with high ion product. The plant processes 7200 tonnes annually at 10MPa, 250 ° C, 1.8wt% water addition, and a residence time of 10-20 minutes.
In addition, Namhae Chemical Co., Ltd. of South Korea started a plant to hydrolyze urea, ammeline, ammelide, and biuret in residual water for melamine synthesis into ammonia and recycle it (2001). The processing amount is 3.4 tons / day.
Another company can recycle ε-caprolactam by 80% or more by hydrolyzing the distillation residue generated from the nylon 6 manufacturing process (18MPa, 330 ° C, residence time 20 minutes, water addition amount 2wt%).
The items shown below are also considered to be capable of chemical recycling by hydrolysis.
| chemical bond | Example of chemical recycling | |
| ether [ -O- ] | Resin | Polyacetal, modified polyphenylene, silicone resin, etc. |
| Chemicals | Epichlorohydrin, pentaerythritol, propylene oxide, bisphenol A, etc. | |
| Others | Cellulose, chitin, chitosan, etc. | |
| ester [-COO-] | Resin | Polyethylene terephthalate, polyurethane, polycarbonate, polybutylene terephthalate, alkyd resin, etc. |
| Chemicals | Dimethyl terephthalate, terephthalic acid, dibutyl phthalate, etc. | |
| amide [ -NH-CO- ] |
Resin | 6-nylon, 6,6-nylon, urea resin, etc |
| Chemicals | TDI, MDI, caprolactam, melanin synthesis residue (urea, Biuret, Ammeline, Ammelide), etc. | |
| Others | Silk, etc | |

When decomposing using a radical reaction in hydrothermal treatment, higher temperature conditions are required than in the case of hydrolysis in Q.11.
For example, when decomposing harmful substances, oxygen is added to supercritical water.
There is also lightening and gasification using radical reactions in supercritical water without using oxygen.
In SuperCriticla Water Oxidation (SCWO), supercritical water mixes with oxygen and the oil to be decomposed, making the reaction extremely easy. Utilizing this, persistent organic chlorine compounds such as PCBs and dioxins are almost completely decomposed. Applying this, Namhae Chemical Co., Ltd. of South Korea started operation of a plant that treats waste liquid in the explosive (DNT / MNT) manufacturing process at 2 m³/hour (waste liquid COD 50000 to 40ppm, total nitrogen 20000 to 60ppm).
The following operating plants have been put into operation in Japan as well.
Pilot plant for sewage sludge treatment (moisture content of 90% or more): 1.1 m³ / hour, 27 MPa, ~ 600 ° C [Kobe City, Hyogo Prefecture (1999)]
Semiconductor manufacturing waste liquid treatment: 25MPa, ~ 600 ℃ [Tateyama City, Chiba Prefecture (1998)]
→ The decomposition rate of TMAH, ammonia, and TOC is 99.9% or more. This can be reused as water used in the factory.
In the example of lightening and gasification, when polypropylene (PP) is treated with supercritical water for 30 minutes, the decomposition rate is reported to be 30% at 400 ° C, 70% at 415 ° C, and 95% at 430 ° C. When the processing time is 60 minutes, the decomposition rate is almost 100%. According to this report, PP becomes molten at high temperatures, and the polymer backbone undergoes thermal decomposition at random positions in the molten phase, initiating a radical reaction. Furthermore, decomposition products of a certain molecular size or smaller are diffused and dissolved in supercritical water, and reactions such as backbone cleavage, hydrogen abstraction, isomerization, cyclization, and radical-to-radical recombination proceed. The product that has been decomposed to the extent of an oligomer remains as an oil phase component. When the main chain end or side chain of PP is cut and further decomposed, it becomes a gas component.
When hexane, which is the main component of naphtha, was used as a raw material and treated with supercritical water at 25 MPa, 380 ° C, and a water addition rate of 0.67, the recovery rate of methane was 78 mol% or more.
Apparatus
In Japan, the High Pressure Gas Safety Act does not apply to the MOMI-cho series. However, under certain conditions, such as the introduction of gas, there is a possibility of being subject to legal regulations. Please contact us for details.
General
Please contact us.
“Supercritical fluid NET” operates the following website (Japanese).
