In large systems, carbon dioxide is recycled. As shown in the flow chart of A.12, carbon dioxide flowing from the extractor is generally reduced to 4 to 6 MPa or less (below the critical point). At this time, the temperature drops and it becomes two phases, a gas phase and a liquid phase. Then heat it to a gas and the dissolved substance will separate. The remaining carbon dioxide gas is stored in the storage tank after being liquefied by the cooler. This liquefied carbon dioxide is cooled further (about 5-10 ° C below the boiling point) to prevent it from vaporizing. It is then pressurized by a circulation pump and fed back to the extractor.
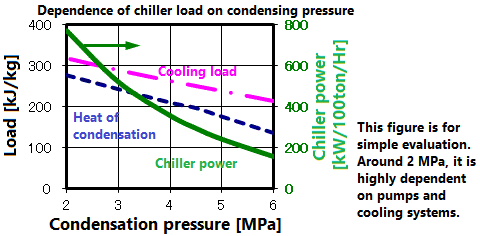
This figure shows the heat of condensation and the load and power of the cooler as a function of pressure. The lower the pressure, the more energy is needed to turn the gas into a liquid. Also, the lower the temperature, the lower the operating efficiency of the cooler. For example, the heat of vaporization at 6MPa and 2MPa is doubled, while the power consumption is about 5 times. That is, efficiency is significantly reduced at low pressures. In the actual design, the terms of use with the separator are also taken into consideration.