The solubility of a substance depends on temperature and density of carbon dioxide. At low density, it exhibits gaseous properties, and solubility decreases.
Therefore, higher pressure is generally better. However, large-scale equipment for pressures above 16 MPa requires different piping and other components, which can significantly increase the cost. Therefore, testing at the upper limit of 16 MPa is also common (the pressure resistance of the testing machine itself exceeds this limit).
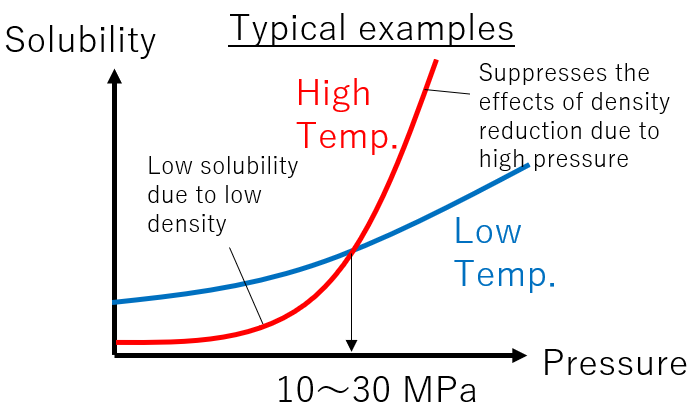
Temperature is more complex. In general, the solubility of a substance increases with increasing temperature at a constant “density”. However, at a constant “pressure”, increasing temperature can lead to a decrease in density and, consequently, a decrease in solubility. A typical example is illustrated in the diagram. In many cases, the slope of increased solubility due to pressure rise becomes more significant at higher temperatures. This means that there is a pressure (10-30 MPa) at which the solubility at high temperatures exceeds the solubility at low temperatures.
Materials near miscibility with carbon dioxide may exhibit a sharp increase in solubility above a certain density. Therefore, it is sometimes sufficient to test at pressures slightly higher than the critical pressure (in which case, liquid carbon dioxide may also be considered as an option).
However, besides solubility, the effect of diffusion must also be considered. In the case of biological materials, it is often necessary to grind the material, which accelerates the diffusion of substances. While solubility represents the equilibrium state, diffusion relates to the rate of dissolution, which affects extraction time. Therefore, increasing the temperature is advantageous for accelerating diffusion.
In our test cases, there are instances where higher temperatures are beneficial, as well as cases where lower temperatures are preferable. This complexity arises from the interplay between the chemical properties of the substance and the structural characteristics of the material.
For the above reasons, we recommend fixing the pressure at the maximum of the planned equipment and varying the temperature, sample type, and presence or absence of an entrainer, unless the decision to use an introduction device above 16 MPa is a factor in the judgment.