Supercritical Fluid
A “supercritical fluid” is a fluid in a supercritical state. Fluid here means not solid. When the fluid is water, it is called supercritical water, and when it is carbon dioxide, it is called “supercritical carbon dioxide” or “supercritical CO2“. In the following we will first briefly describe solids, liquids, and gases to explain what a supercritical state is.
What are solids, liquids, and gases?
Substances have solid, liquid, and gaseous states. For example, water can be “ice, liquid water, or water vapor” and carbon dioxide can be “dry ice, liquefied carbon dioxide, or carbon dioxide gas. What is the difference between solids, liquids, and gases?
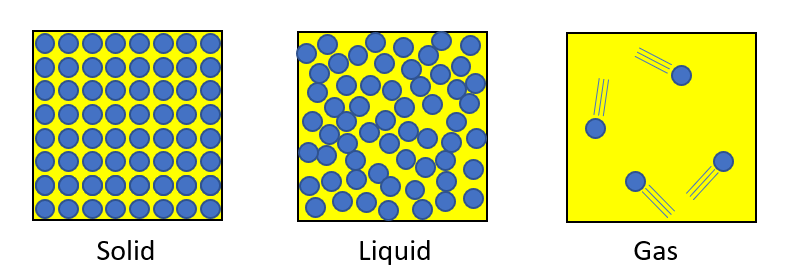
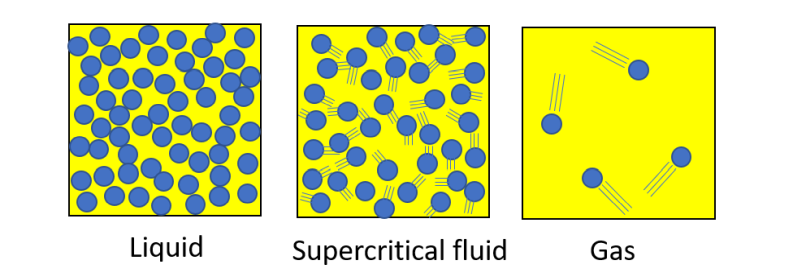
Solid
Molecules are in a regular arrangement. Although the molecules are vibrating, they are still tightly bound to each other and their positions hardly change. Therefore, solids have shape.
Liquid
Molecules are densely packed together in the same way as in solids, but the bonds between molecules are constantly being replaced, so they are never in a fixed position. For this reason, a liquid can always change its shape, even though it is dense like a solid.
Gas
Because the molecules are not bound to each other, they are essentially free in motion and their density is low.
When the energy is high enough to break the bonds between molecules, i.e., as temperature increases, the change from solid to liquid and then to gas occurs. On the other hand, as the distance between molecules becomes closer, the attractive force between molecules becomes stronger up to a certain distance, gases tend to become liquids when the pressure is high (Incidentally, when the pressure is very low, gases sublimate directly from solids without passing through the liquid state. For example, this is the phenomenon of dry ice sublimating to carbon dioxide gas under atmospheric pressure.).
Thermodynamically stable states, such as a solid, a liquid, or a gas, is called “phase”.
How does “phase” change with temperature and pressure?
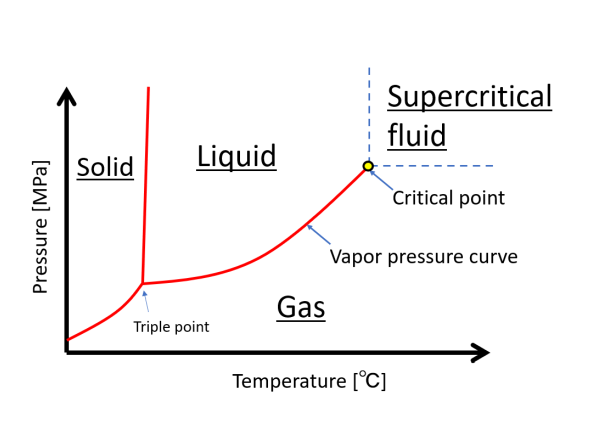
A temperature-pressure “phase diagram” is used to identify the thermodynamic phases at a given temperature and pressure and where the boundaries of these phases are located. A typical example is illustrated below. In the figure on the left, the boundary line between a liquid and a gas is the vapor pressure curve. A substance becomes a gas at a higher temperature or lower pressure than the vapor pressure curve. Since the vapor pressure curve is the boundary between a liquid with dense molecules and a gas with sparse molecules and free motion, the conditions on both sides of the curve are completely different.
This vapor pressure curve is interrupted at the “critical point”. The temperature and pressure at the critical point is called the critical temperature and critical pressure, respectively.
When the pressure is above the critical pressure and the temperature is above the critical temperature, it is called “supercritical fluid. That is, the area to the upper right of the blue dotted line in this figure.
The disappearance of the vapor pressure curve at supercritical conditions means that there is no discontinuous phase change. In other words, the boundary between a supercritical fluid and a liquid or gas is the dotted blue line, but the state at this line only changes continuously. For example, when a liquid is changed from a liquid to a gas via the supercritical state, it becomes a gas without the phenomenon of boiling.
Changes on the phase diagram when temperature or pressure is varied
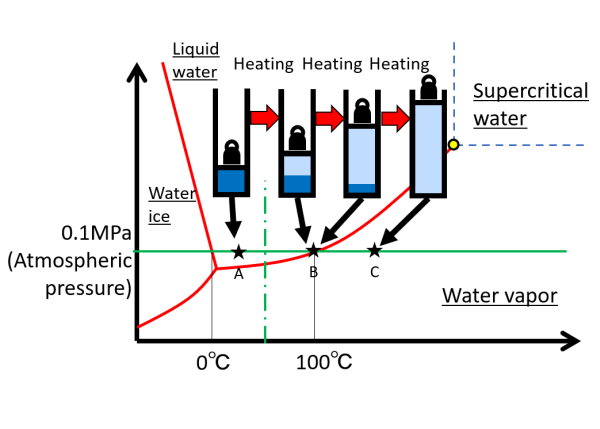
Here, we consider the phase change using the phase diagram. We take water at atmospheric pressure as an example. First, the water-filled piston is under atmospheric pressure (represented by the weight in the right diagram). All water is liquid in state A. When water is heated, its temperature rises and reaches B on the vapor pressure curve. Further heating changes liquid water to vapor, but the temperature remains at 100°C because the thermal energy is consumed as heat of evaporation. This is the phenomenon of boiling. When all the water is vaporized by heating, the temperature rises again over 100 ° C. As shown conceptually in the figure, the volume expands by a factor of 1,700 and the density becomes 1/1,700. The same is true for a change in pressure at a constant temperature (vertical dash-dotted line).
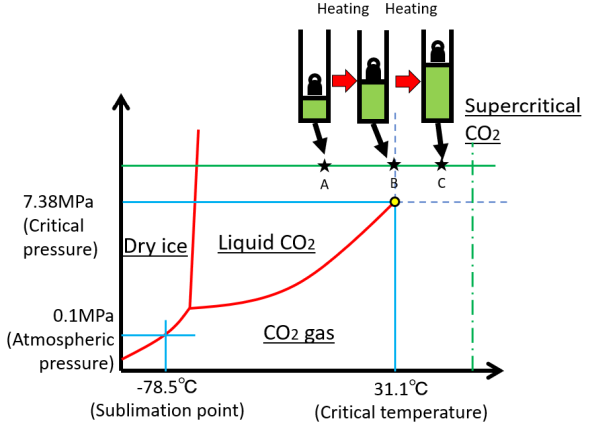
Next, we consider heating above a critical pressure. Now take carbon dioxide as an example. The solid line in the left figure never passes through the vapor pressure curve from state A to C, so the temperature rises and expands smoothly without staying in state B. At this time, phase separation does not occur.
The same is true for pressure changes above the critical temperature (vertical dash-dotted line).
A supercritical fluid is similar to a high-density liquid, but the intensity of molecular motion is similar to that of a gas, and the bonding between molecules is weaker than that of a liquid. Under the low-temperature, low-pressure conditions we normally see, a fluid with properties intermediate between those of a liquid and a gas cannot exist thermodynamically, whereas under high-temperature, high-pressure conditions, there is no distinction between liquid and gas, and a fluid in an intermediate state that suits the purpose can be formed by controlling temperature and pressure.
As a side note, liquids with conditions close to the supercritical fluids are called subcritical fluid. The upper temperature limit of the subcritical state is the critical temperature. There is no clear scientific definition for the lower limit. In the case of water (subcritical water), if the desired chemical reaction is virtually impossible with hot water under atmospheric pressure and is possible at a higher temperature (but not exceeding the critical temperature), it may be conventionally called “subcritical water”.
Examples of critical temperatures and pressures
The supercritical state applies not only to water and carbon dioxide, but also to other substances. Below are examples of critical pressures and critical temperatures. It shows that the inside of a full carbon dioxide cylinder becomes supercritical in the summer, while a hydrogen or nitrogen cylinder is supercritical at room temperature.
| Critical temperature [℃] | Critical pressure [MPa] | |
| Water | 374.1 | 22.1 |
| Carbon dioxide | 31.1 | 7.4 |
| Ethanol | 243.1 | 6.4 |
| Ammonia | 132.4 | 11.3 |
| Hydrogen | -239.9 | 1.3 |
| Nitrogen | -147.1 | 3.4 |
| Mercury | >1400 | >170 |
Supercritical water, Supercritical CO2
For details on each, please refer to the links below.
Supercritical CO2 has applications in extraction, cleaning, drying, dyeing, plating, and particle production, mostly through changes in solubility.
Supercritical water provides a reaction field that cannot be obtained by dry processes. Since reactions proceed efficiently, applications include various types of synthesis, surface treatment, and chemical decomposition.
