Some examples are shown in Q.4. The most typical example is surface tension-free drying.
When treating microstructures, porous materials, etc. with solutions, a drying process is essential for the treated object in a wet state. In such cases, microstructures immersed in the solvent are subjected to the following phenomena.
- If flexible, the structure shrinks without compensating for the decrease in solvent as evaporation progresses
- Subsequently, as drying progresses and the surface of the structure is exposed, an interface between the gas and liquid phases appears inside the structure, and surface tension causes the structure to contract stress toward the tangential direction of the interface
These not only significantly affect the density, microstructure, shape, and physical properties of the material, but also make it more susceptible to cracking/cracking.
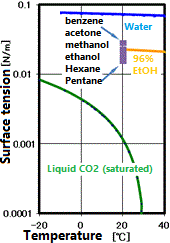
In supercritical drying, the liquid phase can be transferred to the gas phase via the supercritical state. During this time, the interface between the liquid and the gas never appears, and the surface tension that causes the destruction of the microstructure does not occur. This results in drying without shrinkage or damage to the object. Application examples include drying of fine resist patterns of semiconductors, drying after removing the sacrificial layer of MEMS (Micro Electro-Mechanical System), and drying of silica airgel (sold as a high insulation sheet). However, even with supercritical drying, in the presence of other solvents, an interface may be formed and surface tension may occur.