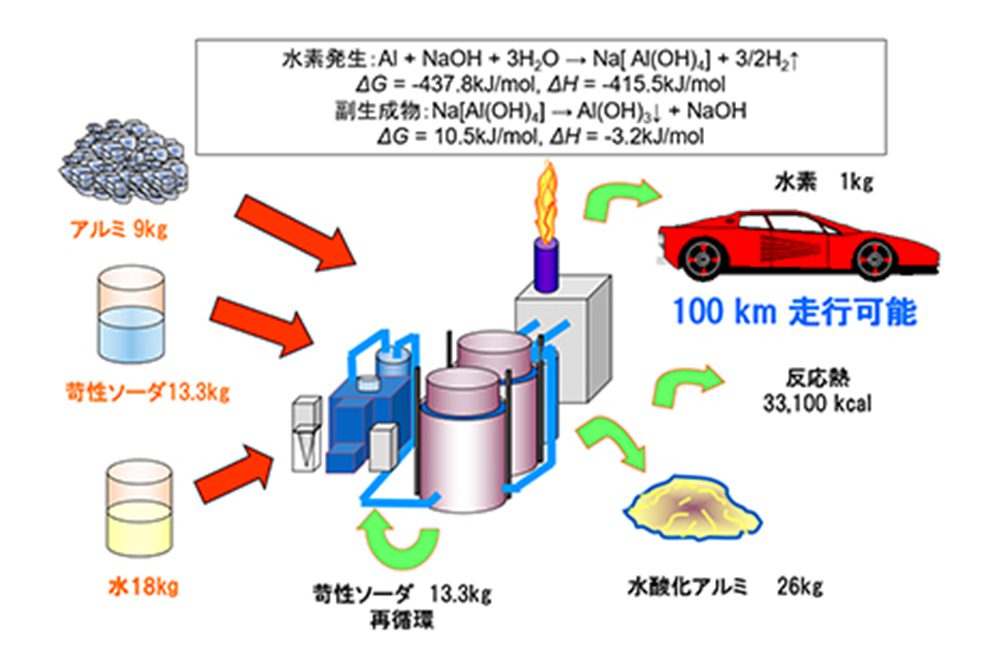
InitiativesToward an earth-friendly future
Toward an earth-friendly future, we are contributing to society and the environment through three initiatives.

test roomTrial test
Supercritical water or hydrothermal treatment, supercritical CO₂ extraction, unhydrous cleaning with CO₂, etc