HOME > Material R & D > Supercritical water
Technical data
What is the supercritical fluid
.
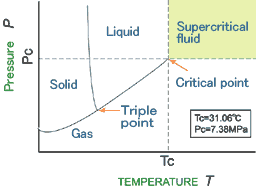 Each compound have the critical point with the specific values of the temperature and the pressure.
The supercritical fluid is defined as the condition exceeding the temperature and the pressure of the critical point.
In this condition, discontinuity between the liquid and the gas phase does not exist.
This means that the supercritical fluid have the property of the diffusivity of the gas and the solubility of the liquid simultaneously.
Figure shows the phase diagram of the carbondioxide as an example.
Each compound have the critical point with the specific values of the temperature and the pressure.
The supercritical fluid is defined as the condition exceeding the temperature and the pressure of the critical point.
In this condition, discontinuity between the liquid and the gas phase does not exist.
This means that the supercritical fluid have the property of the diffusivity of the gas and the solubility of the liquid simultaneously.
Figure shows the phase diagram of the carbondioxide as an example.
.
The property of the supercritical fluid
◎Density is as high as the liquid
◎Diffusion constant and viscosity which correlate to the mobility of the molecule are closer to the gas rather than the liquid
◎Each molecules can be easily interact in the high density fluid like the liquid.
◎In general, higher density causes higher solubility, i.e. good extraction efficiency
We provide the system that can be applied to fields such as washing, synthesis of fine particles, extraction and dyeing by using the supercritical fluid
◎Density is as high as the liquid
◎Diffusion constant and viscosity which correlate to the mobility of the molecule are closer to the gas rather than the liquid
◎Each molecules can be easily interact in the high density fluid like the liquid.
◎In general, higher density causes higher solubility, i.e. good extraction efficiency
We provide the system that can be applied to fields such as washing, synthesis of fine particles, extraction and dyeing by using the supercritical fluid
.
